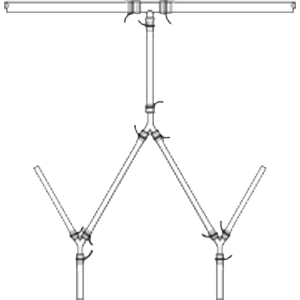
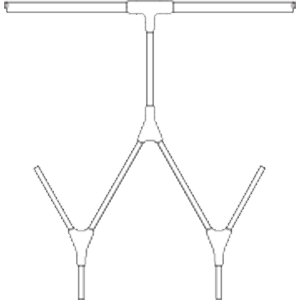
Single-use molded manifolds are custom made to meet your product sampling and storage needs in
drug and vaccine production.
Manufactured from AdvantaFlex® biopharmaceutical grade TPE or AdvantaSil® platinum-cured Class VI silicone, molded manifolds eliminate the need for barbed fittings while providing a seamless transition from tubing to connection for a continuous, unrestricted flow. Designs range from simple molded sanitary ends to complex configurations with varying lengths of tubing and several molded connections.
Single use molded manifolds are not intended for implantation or continuous steam applications.
KEY FEATURES
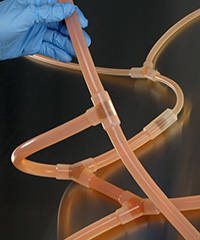


- Eliminates leaks, entrapment, and contamination issues associated with barbed fittings
- Custom manufactured with Y’s, T’s, crosses, reducers, mini and
standard Tri-Clamps®, stoppers and container closures, other shapes like elbows, and tubing - Components are permanently molded into one, Single-Use manifold
- Provides a smooth inner surface without seams or crevices for even, uninterrupted flow
- Reduces end-user component inventory and assembly labor time and expense
- One animal-derived-ingredient-free material contact surface throughout the assembly
- Single use reduces the risk of cross contamination
- Reduces downtime by eliminating certain cleaning validation requirements
- Provides flexibility in facility design—changes are less costly than with hard piping
- Sterilizable by autoclave and gamma radiation—available with validated sterility assurance of 10-6 log reduction per ISO 11137 method VDmax.
- Each material retains its temperature resistance properties after molding
- Imparts no taste or odor to critical streams
- Molded and packaged in ISO Class 7 clean rooms
- Documented lot traceable with identification on bags
- Documented quality control per ISO 9001:2015
- Experienced engineers available for technical and design assistance—benefit from tooling design and development, fast prototyping, and testing capabilities
- DocuLink® (patent pending) manifold assembly identification permanently molds a part number and/or lot number onto a manifold for quick visual reference and to track the part back to its origins (silicone only)
- Optional RFID tags easily attach to assemblies for identification purposes providing reliable electronic data storage and current status of assemblies from inception to disposal
- Complete validation package available upon request
|
37 COMPONENTS |
1 COMPONENT |
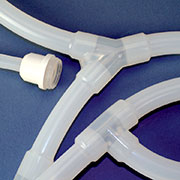
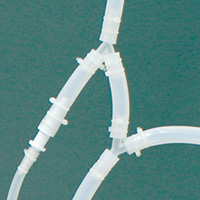
| This example of an assembly made from tubing and barbed fittings is comprised of nine separate pieces of tubing of varying sizes and lengths, plus four fittings that require insertion into the tubing at three points each, with double cable ties installed at each connection for secure attachment. | An AdvantaPure Single-Use Process System is a complete unit, packaged in a sealed polybag and ready for use. Leaks, entrapment, and contamination concerns involved with barbed fitting connections are eliminated. Labor, numerous inventories, and time to assemble the manifold is unnecessary. |
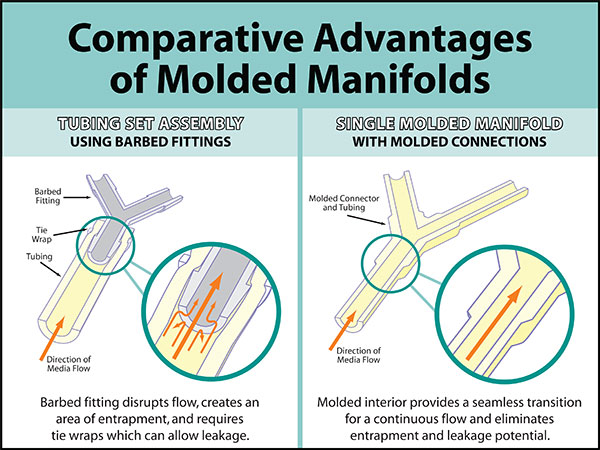
| This illustration of a tubing set assembly using barbed fittings shows how the edge of a fitting may disrupt the flow or create an area of entrapment and potential leakage. Additionally, cable ties used to secure barbed fittings have the potential to pierce bags and packaging. | The interior of a one-piece molded manifold connection provides a smooth inner surface and a seamless transition for a continuous, unrestricted, leak-proof flow. Molded manifolds reduce the risk of a wasted batch of costly product. |
 Molded Connections
Molded Connections
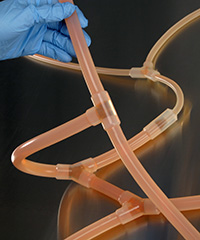
 Single-use Molded Manifolds provide a seamless transition between tubing and connections for a continuous, leak-proof flow. Molded connections include Y, T, cross, reducer, Tri-Clamp®, and mini Tri-Clamp styles. Made from AdvantaSil platinum-cured silicone or AdvantaFlex biopharmaceutical grade TPE, they allow for one material contact surface throughout the system. Connections sizes differ depending on style.
Single-use Molded Manifolds provide a seamless transition between tubing and connections for a continuous, leak-proof flow. Molded connections include Y, T, cross, reducer, Tri-Clamp®, and mini Tri-Clamp styles. Made from AdvantaSil platinum-cured silicone or AdvantaFlex biopharmaceutical grade TPE, they allow for one material contact surface throughout the system. Connections sizes differ depending on style.
Size range: .125″ to 1″ I.D. (3.18mm to 25.40mm I.D.)
MORE ABOUT MOLDED SILICONE CONNECTIONS
MORE ABOUT MOLDED ADVANTAFLEX (TPE) CONNECTIONS
 AdvantaSil® Silicone Tubing and Hose
AdvantaSil® Silicone Tubing and Hose
 AdvantaSil® platinum-cured silicone tubing (called APST) is cleanroom produced and has undergone extensive physical, chemical, and biological testing. They meet many USP standards, including Class VI, as well as FDA, ISO, and European Pharmacopoeia standards. Complete validation packages are available on request.
AdvantaSil® platinum-cured silicone tubing (called APST) is cleanroom produced and has undergone extensive physical, chemical, and biological testing. They meet many USP standards, including Class VI, as well as FDA, ISO, and European Pharmacopoeia standards. Complete validation packages are available on request.
Size range: .030″ through 1″ I.D. (.76mm through 25.40mm I.D.)
MORE ABOUT APST SILICONE TUBING
 AdvantaFlex® BioPharmaceutical Grade TPE Tubing
AdvantaFlex® BioPharmaceutical Grade TPE Tubing
 AdvantaFlex addresses the need for a flexible, translucent, sterilizable, heat sealable, weldable and moldable biopharmaceutical tubing for fluid processing. The tubing is made from FDA-approved ingredients and meets various ISO and USP standards, including Class VI, and European Pharmacopeia standards. A complete validation package is available, and sixteen sizes are stocked.
AdvantaFlex addresses the need for a flexible, translucent, sterilizable, heat sealable, weldable and moldable biopharmaceutical tubing for fluid processing. The tubing is made from FDA-approved ingredients and meets various ISO and USP standards, including Class VI, and European Pharmacopeia standards. A complete validation package is available, and sixteen sizes are stocked.
Size range: 1/8″ to 1″ I.D. (3.18mm to 25.40mm I.D.)
MORE ABOUT ADVANTAFLEX BIOPHARM TPE TUBING
Attach Your Manifold System to a Variety of Sampling and Storage Containers With:
 BioClosure® Systems – High Purity Container Closures
BioClosure® Systems – High Purity Container Closures
 AdvantaPure’s BioClosure Systems offer purity, secure seals, and versatility in design. Stoppers, caps, True Union and GL45 inserts, and other styles, available with or without tubing inserts, are manufactured from platinum-cured, Class VI silicone or AdvantaFlex biopharmaceutical TPE.
AdvantaPure’s BioClosure Systems offer purity, secure seals, and versatility in design. Stoppers, caps, True Union and GL45 inserts, and other styles, available with or without tubing inserts, are manufactured from platinum-cured, Class VI silicone or AdvantaFlex biopharmaceutical TPE.
MORE ABOUT BIOCLOSURE® HIGH PURITY CONTAINER CLOSURES


 BioClosure System Assemblies
BioClosure System Assemblies
 Standardized configurations for media bottles, flasks and carboys using AdvantaFlex Biopharmaceutical Grade TPE tubing and molded components are available.
Standardized configurations for media bottles, flasks and carboys using AdvantaFlex Biopharmaceutical Grade TPE tubing and molded components are available.
MORE ABOUT BIOCLOSURE SYSTEM ASSEMBLIES
 Filling Assemblies
Filling Assemblies
Sterile molded filling assemblies from AdvantaPure continue the shift to Single-Use processes that save time and reduce the risk of cross contamination while increasing productivity between batches. Manufactured with a focus on quality, the assemblies can be quickly installed and operational in a fraction of time as compared to traditional systems used in pharmaceutical and biotech filling.
The multiport Tri-Clamp® design reduces potential leak points, minimizes holdup volume and provides seamless flow.
MORE ABOUT MOLDED FILLING ASSEMBLIES
Tubing Sets
If you’re not sure that molded manifolds are right for your application, let us manufacture your tubing sets (line sets). Assembled in ISO Class 7 clean rooms, they’re fabricated from tubing, fittings, and accessories that you specify, such as filters, aseptic connectors and bottles.
Contact your AdvantaPure sales representative for information.
Experienced engineers with 20 years of knowledge in silicone extrusion and molding are available for technical assistance. Contact your AdvantaPure representative today to see how Single-Use Molded Silicone Manifolds can solve your needs: 1-888-755-4370
![]() Download a printable version of our Single-Use Molded Manifolds brochure. Single_Use_Manifolds.pdf (476KB)
Download a printable version of our Single-Use Molded Manifolds brochure. Single_Use_Manifolds.pdf (476KB)
![]()


The cutaway view above of a molded Y connector shows the smooth interior created by the molding process.

This cutaway of a plastic barbed Y fitting shows the potential areas of flow disruption and entrapment created by the barbs.
![]()
OPTIONS
DocuLink® – Manifold Identification System
DocuLink is our new silicone manifold identification system providing individual identification and tracking information directly on each manifold in the form of a silicone-encased label. Manifold information can be linked to an on-line data management site to provide easy document retrieval for audits and validations. READ MORE…

Gamma Irradiation Services
Have your single use system gamma irradiated before it arrives at your location and save the additional inventorying, transportation costs, and transit time.
Bagged and labeled products are picked up from AdvantaPure’s facility and conveyed to a local gamma irradiation service provider. Using Cobalt 60 radiation, microorganisms and bacteria are destroyed while your single use system remains in its original packaging. Products are then returned to AdvantaPure for final inspection and shipment to your location.
A number of AdvantaPure’s silicone and AdvantaFlex products, particularly those designed for single use, are available with validated sterility assurance of 10-6 per ISO 11137 method VDmax. Contact us for more information at 1-888-755-4370.
| Home | Products | News & Events | About Us | Find a Distributor | Information Request | Contact Us |
| Tubing & Hose | Fittings & Clamps | Single-Use Systems | Molded Products | Custom Products & Services | Hose Identification |
| Product Videos
| Legal Notices & Trademarks | Terms & Conditions of Sale | Privacy Policy | Feedback/Questions | Site Map |
AdvantaPure products are produced by NewAge® Industries, Inc., 145 James Way, Southampton, PA 18966 USA
Phone: 215-526-2151 • 888-755-4370 (toll free US only); Fax: 215-526-2167 • 888-258-4293 (toll free US only); Email: info@www.advantapure.com
©2001-2021 NewAge® Industries, Inc. All rights reserved worldwide.