Phone: 1-888-755-4370 (toll free US only)
Phone: 1-215-526-2151 (worldwide) Email: info@www.advantapure.comRFID Tracking Solutions
Verigenics® is a division of NewAge® Industries Inc. (www.verigenics.com). Previously marketed under NewAge Industries’ AdvantaPure® high purity products line, Verigenics offers solutions to enable medical device, pharmaceutical and other regulated companies to track and authenticate consumables, assets and equipment. Verigenics products use RFID (radio frequency identification) technology to provide enhancements such as increased safety for providers and patients, brand loyalty, increased productivity, inventory visibility and improved product security.
Verigenics RFID products consist of:
- GammaTag® RFID labels and tags that attach to single use medical devices before gamma radiation sterilization
- Hose Track®, an identification and hose lifecycle analysis system offering 21 CFR Part 11 validation
- P•E•T Process Equipment Tracking®, a system similar to Hose Track that applies to process equipment such as filters, storage vessels, and valves
- A variety of software solutions and RFID reader/writers to integrate with tags and labels
- Durable tags able to withstand the most demanding conditions

GammaTag® RFID tags from Verigenics are the first radio frequency identification tags that handle gamma radiation with no loss of data. GammaTag provides reliable electronic data storage of single-use medical devices, bioprocess components and other parts from inception to disposal. GammaTag is available exclusively from Verigenics.
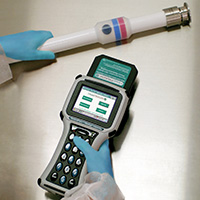
RF IDENTIFICATION AND HOSE LIFECYCLE ANALYSIS SYSTEM
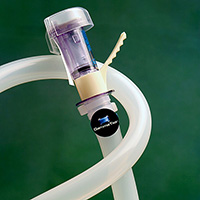
 Hose Track® – A unique hose assembly tracking system that helps minimize the risk of contamination, hose failure, and the costly repercussions associated with batch waste and production stoppages. Provides a 21 CFR Part 11-compliant audit trail to assist in the validation process.
Hose Track® – A unique hose assembly tracking system that helps minimize the risk of contamination, hose failure, and the costly repercussions associated with batch waste and production stoppages. Provides a 21 CFR Part 11-compliant audit trail to assist in the validation process.
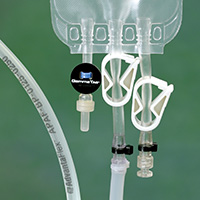
Using Hose Track technology for P•E•T Process Equipment Tracking®, all process components involved with a particular batch of product can be monitored from start to finish. Track usage and cleaning cycles to ensure timely maintenance and replacement . . . before parts begin to fail, risk product integrity, and waste time and labor. It’s a logical approach to tracking all critical process equipment components.
Verigenics is uniquely positioned to support the identification requirements and emerging technologies of the medical device, pharmaceutical, biologic, and other critical industries. Its staff has the experience, understanding, and ability to help make your project successful. Contact a Verigenics representative for more info on RFID Solutions:
Phone: 1-888-323-5131 (toll free US only) • 215-526-2180 (worldwide); Email: info@verigenics.com • Website: www.verigenics.com